 0
0








 0
0








The growing adoption of advanced medical devices has led to the design and utilization of a diverse range of complex medical equipment, both in hospital settings and for telecare at home. Given the diverse settings and applications of these medical devices, designers face a critical challenge that must be addressed at the outset of the design process: How can medical devices, including their power supplies, be selected, or designed to ensure safe and appropriate use?
This article explains the risk guidelines applicable to medical devices used with patients, the required protective measures for different types of medical devices, the designated usage environments for these devices, and electromagnetic compatibility (EMC) considerations crucial for designing power supplies for medical devices in any setting. These medical device design requirements are mainly set up by IEC 60601-1 and its collateral standard, IEC 60601-1-2.
Governing standards for medical devices and medical-grade power supplies
The most important standard governing the medical devices design is, IEC 60601-1. The standard defines the general requirements for basic safety and essential performance of a medical device. The compliance with IEC 60601-1 is essential to any medical device commercialization in the USA and worldwide, as the European EN and Canadian CSA versions of the standard are identical to the IEC 60601-1 standard. For EMC compliance, a medical device needs to comply with IEC 60601-1-2, Electromagnetic Disturbances – Requirements and Tests, which is a collateral standard to IEC 60601-1.
Basic safety requirements for medical devices and medical grade power supplies per IEC 60601-1
Any medical grade power supply must be designed/chosen in such a way that medical devices’ basic safety and essential safety are not compromised. The IEC 60601-1 ensures patient safety by requiring means of protection, abbreviated as MOP. The MOP is the means for reducing the risk due to electric shock that can be achieved through insulation, air clearances, creepage distances, impedance, and protective earth connections.
The varnishing, enameling, oxidation, and similar protective finishes, as well as covering with sealing compounds that can re-plasticize at temperatures to be expected during operation (including sterilization), shall not be regarded as a means of protection (MOP). The MOP (Figure 1) is further classified into MOOP (means of operator protection) and MOPP (means of patient protection). The design requirements for example creepage and clearance are different for MOOP and MOPP.
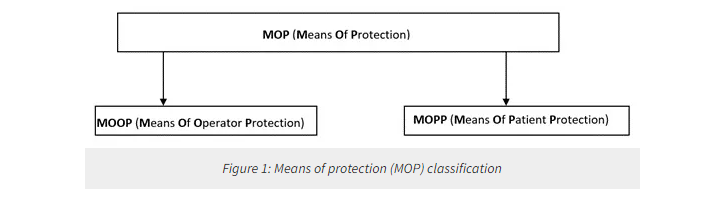
The MOP compliance is checked using the dielectric strength test for solid insulation as MOP. When creepage and air clearance values are claimed as MOP then values shall comply with the limits specified in the IEC 60601-1. When protective earth connections form a means of patient protection, it shall comply with the ground bond test requirements of the standard.
How to choose a power supply based on means of protection MOP
The obvious question for a designer is how to choose a medical-grade power supply based on the means of protection (MOP) requirements. As per IEC60601-1, any medical device hence the power supply should provide medical two means of protection to prevent applied parts and other accessible parts, Figure 3.
The obvious question for a designer is how to choose a medical-grade power supply based on the means of protection (MOP) requirements. As per IEC60601-1, any medical device hence the power supply should provide medical two means of protection to prevent applied parts and other accessible parts, Figure 3.
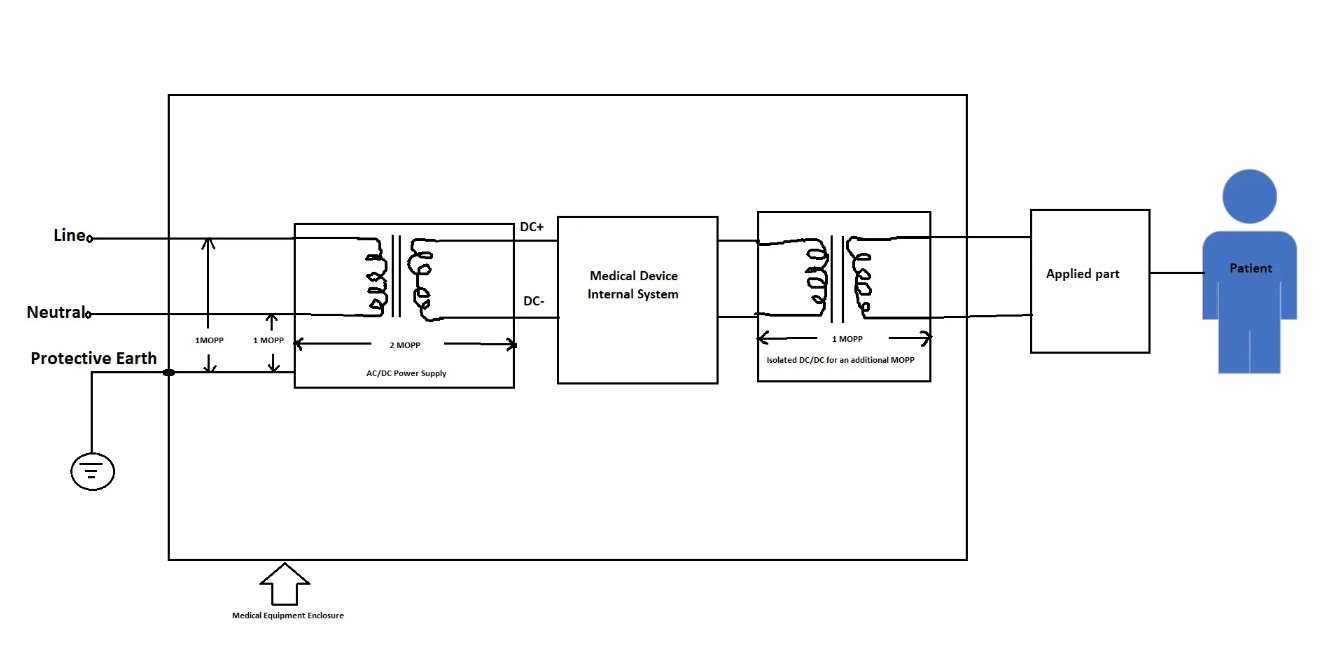
Figure 2: Simplified Insulation/Isolation diagram for a medical device
Role of leakage current while selecting the power supply
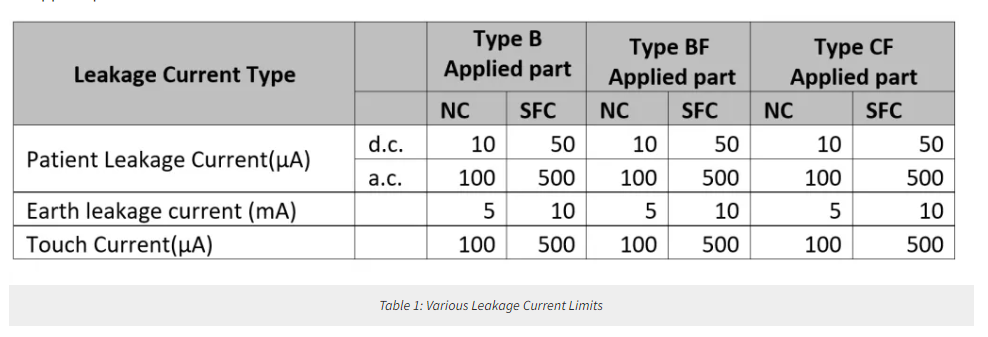
The leakage current is defined in IEC60601-1 as the current that is not functional. The following leakage currents are classified as earth leakage current, touch current, and patient leakage current.
The earth leakage current is the current flowing through the protective earth conductor, but it is not a patient or operator hazard as both are protected by specifying appropriately low values for patient leakage current and touch current in normal conditions and relevant single fault conditions including interruption of the protective earth conductor. However, an excessive earth leakage current could pose a possible problem for the installation’s earthing system and any circuit breakers operated by current imbalance detectors. For permanently installed medical equipment connected to a supply circuit that supplies only the medical equipment, a higher value of earth leakage current is allowed than those in Table 1.
Touch Current is the leakage current flowing from the enclosure or parts thereof, excluding patient connections, accessible to any operator or patient in normal use, through an external path other than the protective earth conductor, to earth, or another part of the enclosure.
Patient leakage current is the current flowing from the patient connections via the patient to earth, or the current originating from the unintended appearance of a voltage from an external source on the patient and flowing from the patient via the patient connections of an f-type applied part to earth.
An important term to understand is the concept of Applied Part, which is part of medical equipment that comes into physical contact with the patient to perform its function. For example, one of the applied parts of an ECG machine is the ECG electrode. The applied part of a medical device can be type B (body), BF (Body Floating), or CF (Cardiac Floating).
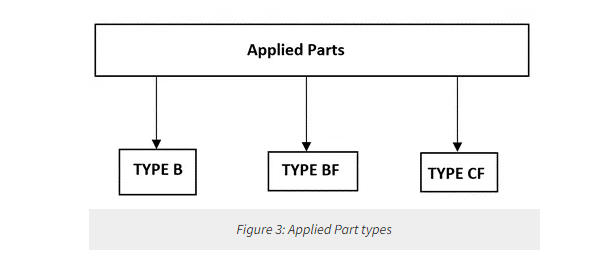
Type B applied provides some protection against electrical shock, but is the least stringent regarding allowable patient leakage current and patient auxiliary current. TYPE B applied parts are not suitable for direct cardiac application. Type BF is electrically floating applied parts complying with the specified requirements of this standard to provide a higher degree of protection against electric shock than that provided by TYPE B applied parts. Type CF is electrically floating applied parts complying with the specified requirements of this standard to provide a higher degree of protection against electric shock than that provided by type BF applied parts.
Sometimes, standard power supplies, including those that are medically approved, do not meet the output-to-ground isolation or patient leakage current requirements. An additional isolation transformer at the input of the power supply or a secondary DC/DC converter may be required to ensure compliance.
Conclusion
There are a lot of power supplies that are listed based on medical grade just because they provide two means of patient protection, but might be missing the additional IEC 60601-1 requirements. Designing or selecting a power supply for medical devices, especially those that involve electrical contact with the patient, requires addressing a dual challenge: minimizing leakage currents under normal conditions and providing ground isolation to protect the patient in case of a fault.
Achieving lower leakage currents, especially for cardiac application (BF rated) presents its own set of challenges. Apart from the necessity to significantly increase internal component spacing on the secondary side to meet high isolation voltage requirements, there is also a conflict between achieving low leakage currents and ensuring low emissions.
It is also crucial to minimize both differential and common-mode noise using a low-noise topology, as well as to reduce line-frequency ripple in the primary circuits. The designer should not aim for a lower leakage current rating than required, as it may result in higher emissions. After the power supply is integrated into the system, the leakage current needs to be measured for the overall system, but having a medical-grade supply gives the designer confidence in achieving compliance with IEC 60601-1.
About US
Heisener Electronic is a famous international One Stop Purchasing Service Provider of Electronic Components. Based on the concept of Customer-orientation and Innovation, a good process control system, professional management team, advanced inventory management technology, we can provide one-stop electronic component supporting services that Heisener is the preferred partner for all the enterprises and research institutions.
